- Home
- About Us
- Students
- Academics
-
Faculty
- Electrical Engineering
- Automation
- Computer Science & Engineering
- Electronic Engineering
- Instrument Science and Engineering
- Micro-Nano Electronics
- School of Software
- Academy of Information Technology and Electrical Engineering
- School of Cyber Security
- Electrical and Electronic Experimental Teaching Center
- Center for Advanced Electronic Materials and Devices
- Cooperative Medianet Innovation Center
- Alumni
-
Positions
-
Forum
News
- · Shanghai Jiao Tong University professors Lian Yong and Wang Guoxing's team have made remarkable progress in the field of high-efficiency pulse neural network accelerator chips.
- · AI + Urban Science research by AI Institute was selected as cover story in Nature Computational Science!
- · The first time in Asia! IPADS's Microkernel Operating System Research Wins the Best Paper Award at SOSP 2023
- · Delegation from the Institution of Engineering and Technology Visits the School of Electronic Information and Electrical Engineering for Journal Collaboration
- · Associate professor Liangjun Lu and research fellow Jiangbing Du from Shanghai Jiao Tong University made important advancements on large capacity and low power consumption data transmission
Guo Gao and co-workers from Shanghai Jiao Tong University achieved significant progress on calcium-ion pre-intercalated hydrated vanadium oxide cathode to facilitate high-performance zinc storage
Recently, the research paper "Revealing the role of calcium ion intercalation of hydrated vanadium oxides for aqueous zinc-ion batteries" of Gao Guo's team, School of Sensing Science and Engineering, School of Electronic Information and Electrical Engineering, Shanghai Jiao Tong University, has been published in “Journal of Energy Chemistry”, an international authoritative journal in the field of energy. In this research, a new type of vanadium oxide cathode based on Ca2+ and Ba2+ intercalation was innovatively constructed, revealing the key roles played by different alkaline-earth metal ions in the pre-intercalation of hydrated vanadium oxides, and through a series of physical characterization, electrochemical performance tests, kinetic analysis, and investigation of the zinc storage mechanism, the excellent zinc storage capacity of CaVO cathode was identified, which makes it an important prospect for energy storage applications.

Fig. 1 Paper published in the Journal of Energy Chemistry
Research background:
As a typical representative of cathode materials for aqueous zinc ion batteries, vanadium-based materials have attracted much attention due to their high specific capacity, open structure, and multivalent states. Among them, vanadium pentoxide (V2O5) based on a two-electron redox center is regarded as an ideal cathode with excellent zinc storage performance. However, low electron/ion transfer kinetics and structural fragility during cycling have led V2O5 to show poor rate capability, which greatly limits its application. To address these challenges, researchers have made considerable efforts to optimize the zinc storage performance of V2O5. One of the modification strategies is to introduce both metal ions and H2O molecules between the V2O5 layers to synergistically enhance the structural stability of the material, thus ensuring its fast electrochemical reaction kinetics. However, there is still much room for improvement in the rate capacity and cycling performance of such cathode materials that have been reported so far. Therefore, it is still necessary to further improve their zinc storage performance by new experimental methods. Not only that, the precise effects regarding the electrochemical performance and kinetics of different cationic pre-intercalations of the same main group on the cathode have not been systematically discussed. Given this, the team explored the question of the key role played by different pre-intercalated cations in vanadium oxide cathodes and comprehensively verified their feasibility for zinc storage.
Research highlights:
In this work, CaVO and BaVO materials were synthesized by a simple one-step hydrothermal method, and the whole battery system was discussed in detail through systematic physical characterization, electrochemical performance testing, kinetic analysis, in-depth EIS characteristic parameter discussion, and investigation of the zinc storage mechanism, which further identified the excellent zinc storage capacity of CaVO as well as the energy storage mechanism.
The crystal structure of V2O5 is rearranged based on the reaction between H2O2 and V2O5, which further promotes the intercalation of Ca2+ and Ba2+ (Fig. 2). At this point, its layer spacing becomes smaller after pre-intercalating cations, which is different from the conventional intercalation of Ca2+ and Ba2+, primarily due to the strong interactions between divalent metal cations and lattice oxygens in the V-O bilayers, resulting in the formation of strong metal-oxygen bonds. In addition, synthesizing the formula for calculating the percentage of ionic properties proposed by Pauling and the results of electrochemical performance tests, it can be seen that it is unscientific to judge the energy storage behavior of the system as good or bad based on the strength of the stability of ionic bonding alone. More fundamental studies are needed regarding the exact cause mechanism of its superior performance.
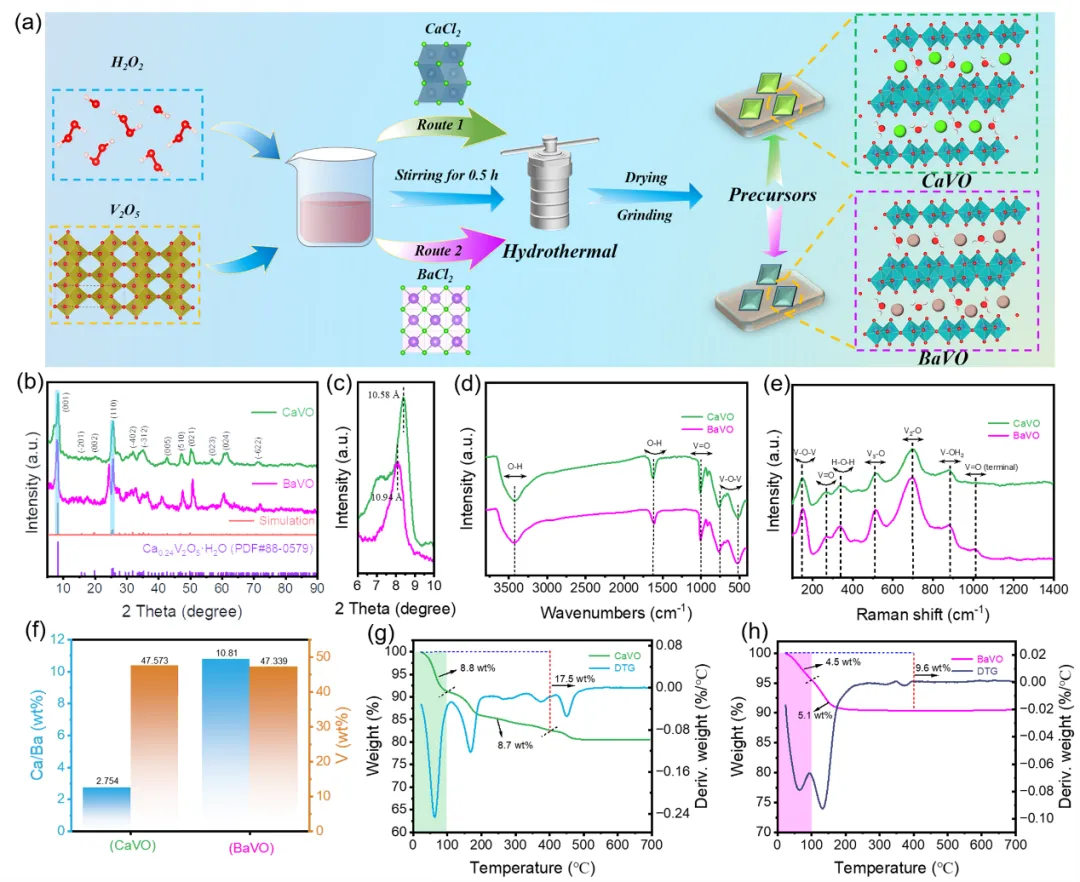
Fig. 2 Physical characterization of the CaVO and BaVO materials
Compared with BaVO, CaVO cathode exhibits excellent overall electrochemical performance (Fig. 3). CaVO presents a discharge-specific capacity of 470.2 mAh g−1 at 0.1 A g−1 for the first cycle, and can be as high as 489.8 mAh g−1 after waiting for cycling to be stabilized, which is much higher than the specific capacity of BaVO (400.6 mAh g−1 in the first cycle) and commercial V2O5 cathodes. In addition, CaVO still demonstrates high rate capacity at different current densities. Even at a high current density of 3 A g−1, CaVO maintains a discharge-specific capacity of 201.2 mAh g−1 after 1000 cycles.
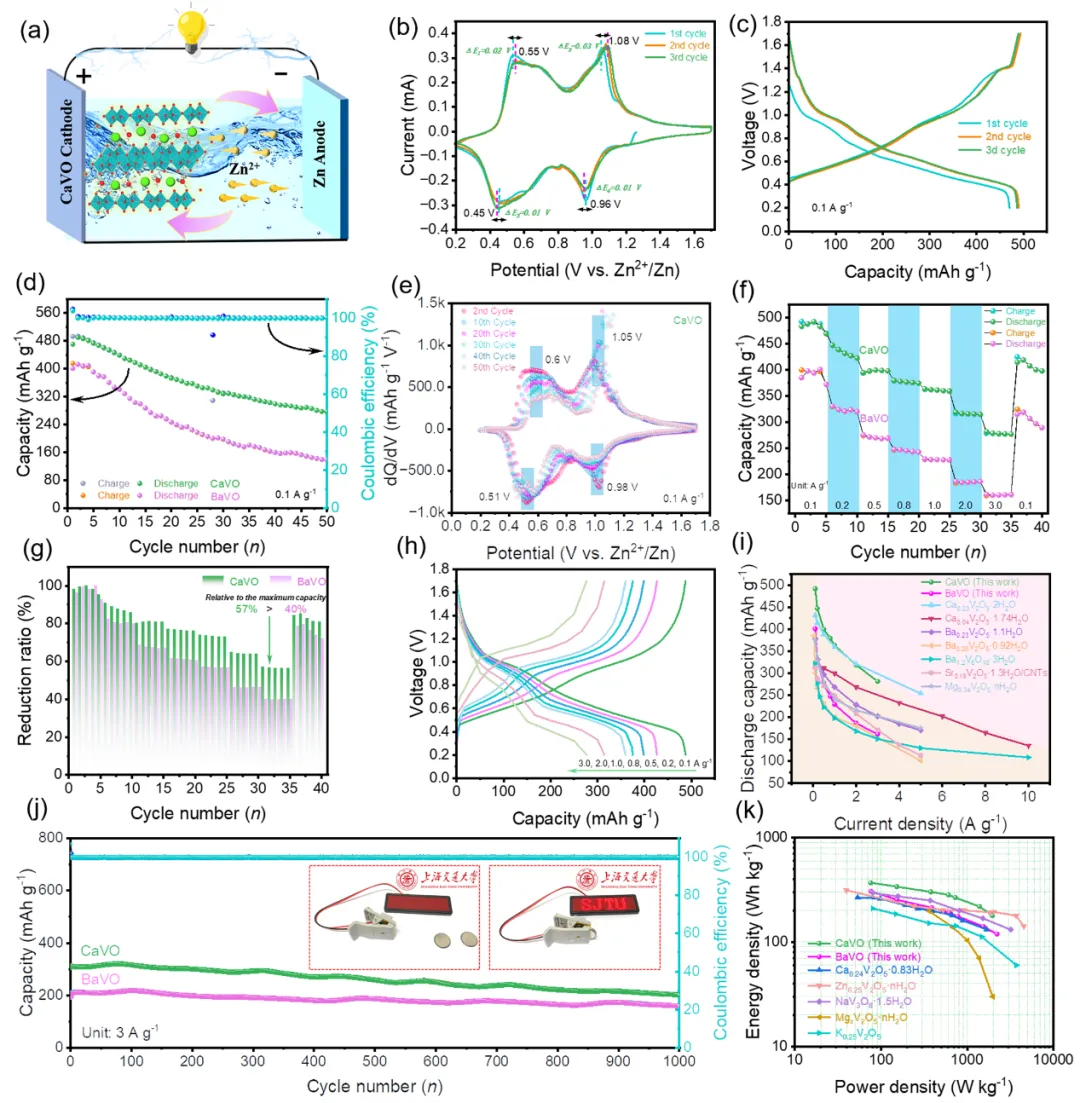
Fig. 3 Electrochemical properties of the CaVO and BaVO cathodes
In terms of electrochemical reaction kinetics, CaVO shows a high pseudocapacitance contribution (0.5 mV s−1, 53.15%), as well as a high diffusion coefficient of Zn2+ (0.99×10−9 cm2 s−1) (Fig. 4). In addition, a shorter Zn2+ diffusion path, lower electronic resistance, and higher electronic conductivity exist in the CaVO//Zn battery system, which is favorable for stable electrochemical reactions in CaVO.
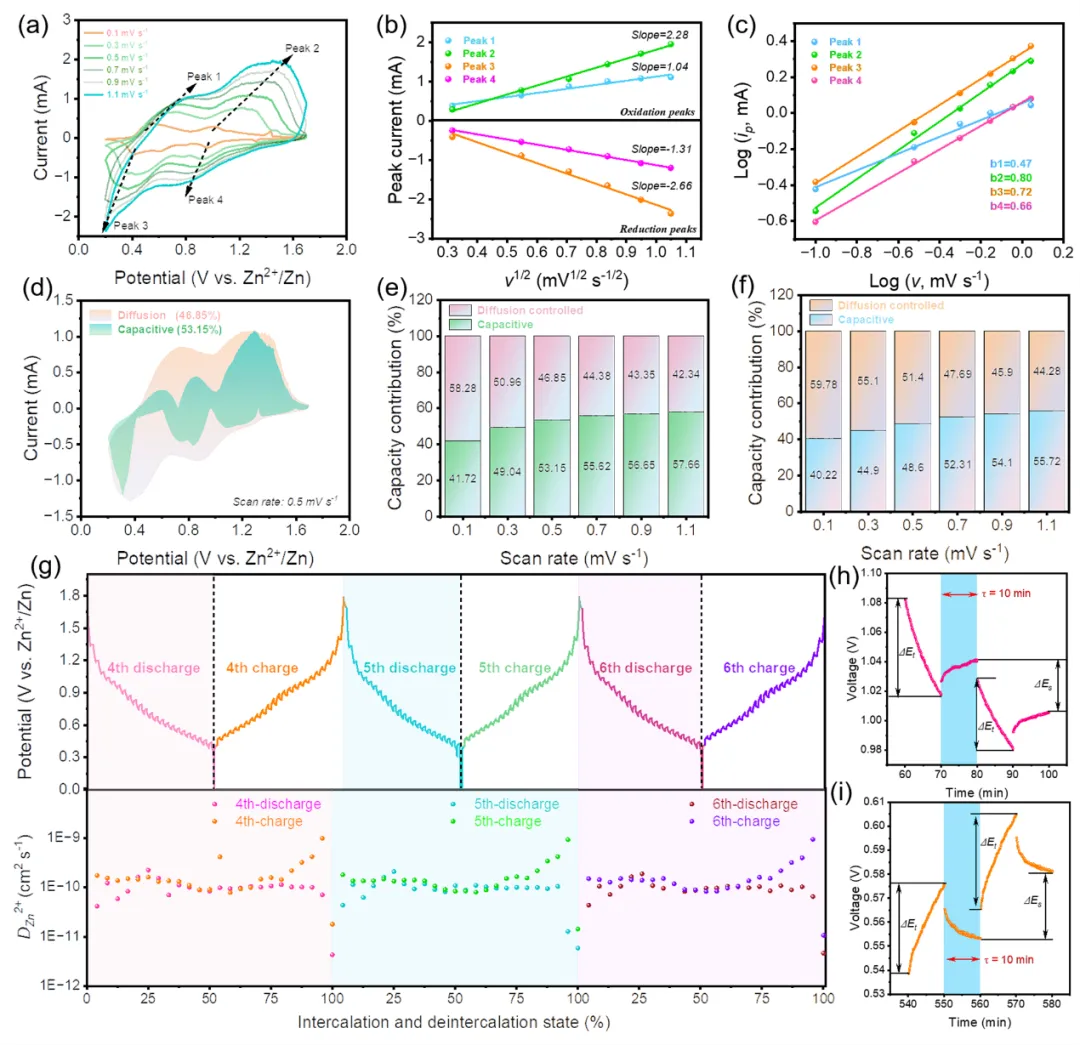
Fig. 4 Electrochemical reaction kinetics performance of the CaVO cathode
Based on the Zn storage mechanism research, it is known that CaVO undergoes reversible expansion and contraction of the interlayer spacing to accommodate the reversible insertion/detachment of Zn2+ and H+ (Fig. 5). In addition, the stability of the CaVO structure stems from the co-stabilized presence of structural water molecules and Ca2+. The structured water molecules can act as a charge shielding "lubricant" to promote the effective diffusion of Zn2+, and the introduction of Ca2+ effectively maintains the crystal structure of the host material and successfully improves the charge transfer kinetics of the battery system.
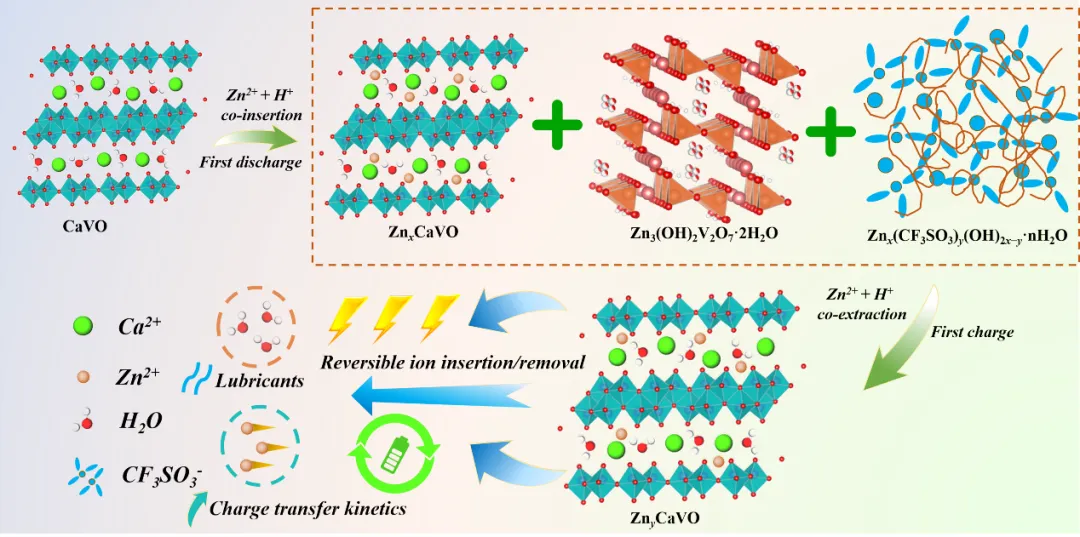
Fig. 5 Zinc storage mechanism of the CaVO cathode
Paper Information:
The first author of this paper is Ph.D. candidate Zhou Tao from the School of Electronic Information and Electrical Engineering at Shanghai Jiao Tong University, with Associate Researcher Gao Guo as the corresponding author. This research was supported by the National Key Research and Development Program of China (2022YFA1207503) and the Giga Force Electronics Interdisciplinary Funding (JJHXM002208-2023).
Paper Link: https://doi.org/10.1016/j.jechem.2024.03.022
-
Students
-
Faculty/Staff
-
Alumni
-
Vistors
-
Quick Links
