- Home
- About Us
- Students
- Academics
-
Faculty
- Electrical Engineering
- Automation
- Computer Science & Engineering
- Electronic Engineering
- Instrument Science and Engineering
- Micro-Nano Electronics
- School of Software
- Academy of Information Technology and Electrical Engineering
- School of Cyber Security
- Electrical and Electronic Experimental Teaching Center
- Center for Advanced Electronic Materials and Devices
- Cooperative Medianet Innovation Center
- Alumni
-
Positions
-
Forum
News
- · Bin Dai's Team Unveils the Assembly Mechanism of β-Lactoglobulin Fibrils, Providing New Insights for the Development of Functional Nanomaterials
- · Mingyi Chen’s research group has made important progress in the field of analog-to-digital converter chips for brain-computer interface
- · Progress in the Development of Semiconductor Nanomaterials to Activate Pyroptosis for Cancer Therapy
- · Jiamiao Yang’s team achieved the high precision optoelectronic reservoir computing based on complex-value encoding
- · Significant Advancements in Resonator-Enhanced Quantum Sensing Achieved by Zenguihua's Team at the School of Sensing Science and Engineering
Progress in the Development of Semiconductor Nanomaterials to Activate Pyroptosis for Cancer Therapy
Recently, Prof. Honglu Zhang's team from the School of Electronic Information and Electrical Engineering (SEIEE), Shanghai Jiao Tong University (SJTU), in collaboration with Academician Chunhai Fan from the National Center for Translational Medicine, Prof. Qianjun He from the School of Materials Science and Engineering, and Associate Prof. Huan Zhang from the School of Agriculture and Biology, has made significant advancements in utilizing novel semiconductor nanomaterials for photocatalytic pyroptosis in cancer therapy. Their findings, titled “Mitochondria-targeted Photoredox Catalysis Activates Pyroptosis for Effective Tumor Therapy” have been published in the prestigious journal Advanced Functional Materials.

Research Background
A major challenge in cancer therapy is achieving efficient destruction of cancer cells while minimizing damage to healthy tissues. Traditional therapies, such as chemotherapy and radiotherapy, have significant side effects and can induce drug resistance. Therefore, scientists are actively exploring safer and more precise alternative technologies. In this study, the research team innovatively designed a two-dimensional molybdenum oxide semiconductor nanomaterial rich in oxygen vacancies (Mt-VR-MoO₃). Utilizing its efficient photocatalytic properties, they achieved targeted cancer therapy through the induction of pyroptosis. This approach pioneers the integration of semiconductor photocatalysis and pyroptosis, offering a new breakthrough in cancer treatment.
Photocatalysis has gained significant attention in cancer therapy due to its non-invasive nature, precision, and controllable light response. However, most existing photocatalysts are limited to the visible light spectrum and suffer from poor biocompatibility and insufficient targeting. Meanwhile, pyroptosis, a form of programmed cell death accompanied by the release of inflammatory factors, can trigger strong anti-tumor immune responses, making it a promising focus in cancer research. However, efficiently and controllably inducing pyroptosis remains a challenge.
This study introduces a defect-rich molybdenum oxide semiconductor material with unique conduction and valence band structures, demonstrating excellent responsiveness in the near-infrared (NIR-II) region. By combining photocatalysis with the pyroptosis mechanism, the study provides an innovative solution for cancer therapy.
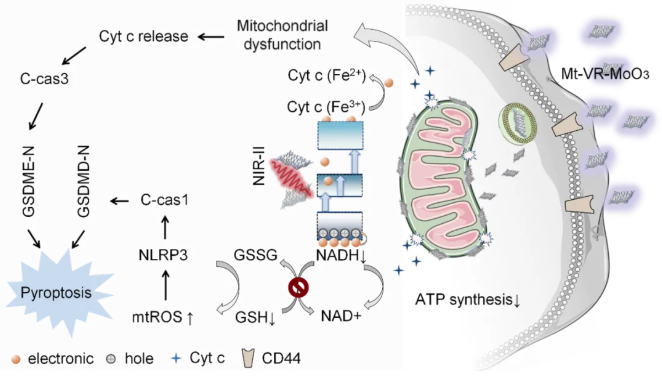
Figure 1. Schematic illustration of mitochondria-targeted, NIR-II-responsive photocatalyst (Mt-VR-MoO₃) activating pyroptosis for tumor therapy
Research Progress
By introducing abundant oxygen vacancies into two-dimensional molybdenum oxide nanosheets (VR-MoO₃), the research team significantly enhanced the material's optoelectronic properties. The oxygen vacancies create intermediate energy bands, enabling efficient electron-hole pair generation under NIR-II (1064 nm) light, driving a series of redox reactions. Additionally, the material was modified with triphenylphosphine (TPP) for mitochondrial targeting and hyaluronic acid (HA) for enhanced biocompatibility, resulting in a mitochondria-targeted photocatalyst (Mt-VR-MoO₃).
Under NIR-II irradiation, Mt-VR-MoO₃ catalyzes the oxidation of mitochondrial NADH and the reduction of cytochrome c (Cyt c), disrupting the mitochondrial electron transport chain (Mito-ETC). This cascade significantly elevates mitochondrial reactive oxygen species (mtROS) levels, reduces mitochondrial membrane potential, opens mitochondrial permeability transition pores (MPTP), and releases cytochrome c into the cytoplasm. These reactions activate Caspase-1 and Caspase-3-mediated pyroptosis pathways, leading to cancer cell rupture.
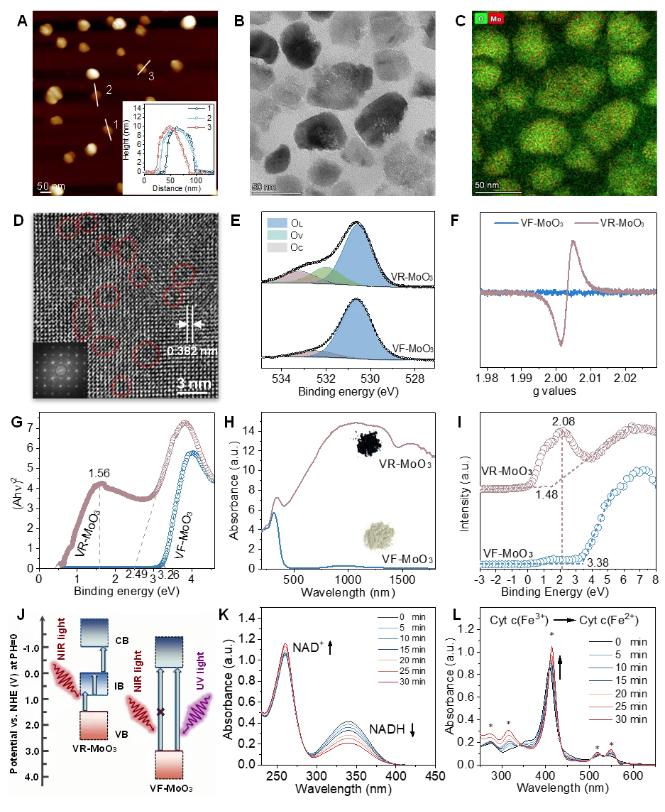
Figure 2. Morphology and photocatalytic performance characterization of the photocatalyst (Mt-VR-MoO₃).
Pyroptosis is unique in its dual role: directly killing cancer cells while releasing inflammatory factors to activate the immune system, further enhancing anti-tumor effects.
In a mouse breast cancer model, the material achieved an 86.9% tumor growth inhibition rate, significantly outperforming traditional therapies. Histological and immunohistochemical analyses showed significantly elevated expression levels of pyroptosis-related proteins (e.g., GSDMD, GSDME) in tumor tissues treated with Mt-VR-MoO₃, confirming successful induction of pyroptosis. Biodistribution studies revealed that the material was rapidly excreted via feces and urine, demonstrating excellent biocompatibility and minimal toxicity to normal organs.
Significance of the Study
This study represents the first successful integration of defect-rich molybdenum oxide semiconductor photocatalysis with pyroptosis induction for cancer therapy. The strategy not only overcomes the wavelength limitations of existing photocatalysts but also enhances anti-cancer effects by activating the immune system through pyroptosis. Most importantly, this therapy relies solely on photocatalysis without the need for exogenous drugs, supporting the concept of "drug-free cancer therapy."
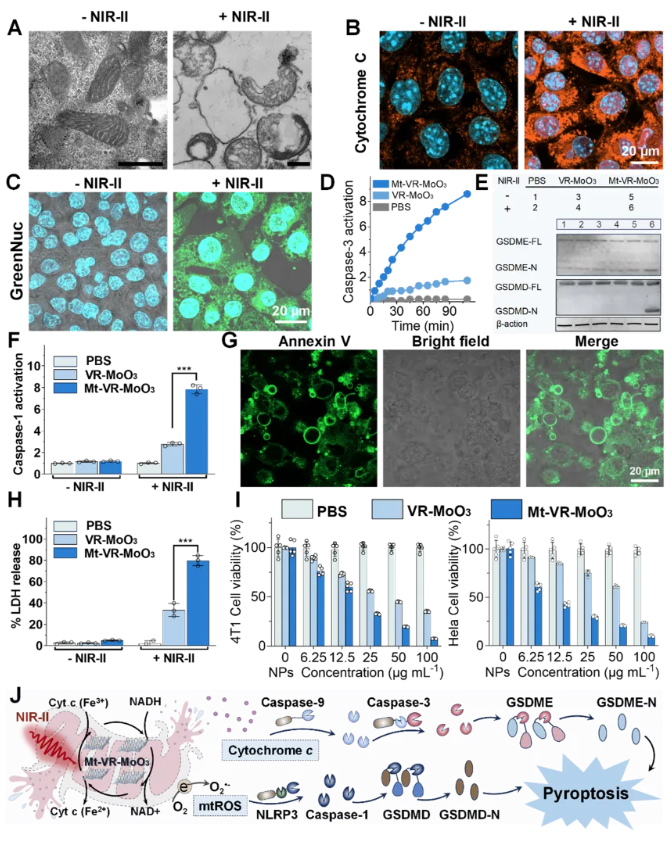
Figure 3. Mechanism of mitochondrial disruption and pyroptosis induction by the photocatalyst (Mt-VR-MoO₃) under near-infrared (NIR) light irradiation, leading to mitochondrial damage and subsequent activation of pyroptosis in cancer cells
About the paper
The study was jointly led by Associate Prof. Honglu Zhang from SEIEE, Associate Prof. Huan Zhang from the School of Agriculture and Biology, and Prof. Qianjun He from the School of Materials Science and Engineering/Hydrogen Science Center. PhD student Bin Zhao served as the first author. The project received valuable guidance and support from Academician Chunhai Fan from the School of Chemistry and Chemical Engineering.
The research was supported by funding from the National Key R&D Program, the National Natural Science Foundation of China, and the Shanghai Pujiang Talent Program.
Prof. Honglu Zhang’s team focuses on using framework nucleic acids for single-molecule biosensing, DNA storage and computation, artificial intelligence, and synthetic biology. Their research findings have been published in prestigious journals such as Nature Communications, PNAS, Matter, and Nature Protocols, with over ten patents filed.
article link: https://onlinelibrary.wiley.com/doi/epdf/10.1002/adfm.202417681
-
Students
-
Faculty/Staff
-
Alumni
-
Vistors
-
Quick Links
